Influence of surface atomic structure demonstrated on oxygen incorporation mechanism at a model perovskite oxide
Category: Scientific HighlightsPublished in Nature Communications
Perovskite oxide surfaces catalyze oxygen exchange reactions that are crucial for fuel cells, electrolyzers, and thermochemical fuel synthesis. Here, by bridging the gap between surface analysis with atomic resolution and oxygen exchange kinetics measurements, we demonstrate how the exact surface atomic structure can determine the reactivity for oxygen exchange reactions on a model perovskite oxide. Two precisely controlled surface reconstructions with (4 × 1) and (2 × 5) symmetry on 0.5 wt.% Nb-doped SrTiO3(110) were subjected to isotopically labeled oxygen exchange at 450 °C. The oxygen incorporation rate is three times higher on the (4 × 1) surface phase compared to the (2 × 5). Common models of surface reactivity based on the availability of oxygen vacancies or on the ease of electron transfer cannot account for this difference. We propose a structure-driven oxygen exchange mechanism, relying on the flexibility of the surface coordination polyhedra that transform upon dissociation of oxygen molecules.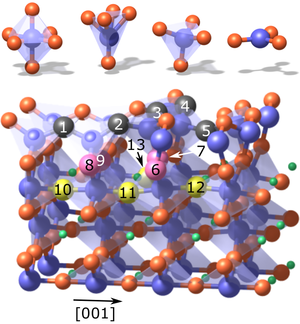
Project part: P15 Collective Phenomena in Oxide Films and Hetero-structures
Original publication: Michele Riva, Markus Kubicek, Yianfeng Hao, Giada Franceschi, Stefan Gerhold, Michael Schmid, Herbert Hutter, Juergen Fleig, Cesare Franchini, Bilge Yildiz, and Ulrike Diebold
DOI: 10.1038/s41467-018-05685-5
Sensengasse 8/12
A-1090 Vienna
AUSTRIA
T: +43-1-4277-51401
F: +43-1-4277-9514


